
All 5 elements are elements that exist in nature. Halogen Is the name Element 7 in the periodic table or halogen element Element in this group There are 5 types of fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astringent (At). Both in reality Production or use of some halogen-free products May not always be beneficial to humans or the environment Because both manufacturers and consumers understand that halogen free Or halogen-free products are considered “green products” that are environmentally friendly. The term “Halogen Free” or “Halogen-free product” is another word that is of interest to all parties, including producers, environmentalists, NGOs and lawmakers. All rights reserved.After the crisis, RoHS regulations, leading manufacturers around the world Have turned to compete for more environmentally friendly products To compete for leadership in the market of environmentally friendly products that are open to all manufacturers with innovations that help sustain the environment Many manufacturers And they searched for environmental issues That is in the current trend of consumers and the public To be used as their selling point Copyright © 2022, Columbia University Press. The Columbia Electronic Encyclopedia, 6th ed.
Some other halogen compounds are calomel (mercurous chloride), fluorite, sal ammoniac ( ammonium chloride), corrosive sublimate ( mercuric chloride), and chlorine bleaches. Chloroform, iodoform, and carbon tetrachloride are halocarbons. They also form halocarbons, compounds with carbon and often other elements such as hydrogen and oxygen. They form numerous metal halides, or salts, e.g., sodium chloride, common table salt. With hydrogen they form hydrogen halides, whose water solutions are called hydrohalic acids, e.g., the water solution of hydrogen chloride is called hydrochloric acid. The halogens form numerous compounds with other elements. Pure halogens exist as diatomic molecules, e.g., Cl 2 they form interhalogen compounds, i.e., compounds between two halogens. Iodine is a grayish black solid and is the least chemically active of the four however, among the nonmetals only oxygen is more reactive than iodine. Chlorine, a yellow-green gas, is more dense and less reactive than fluorine. Fluorine, a pale yellow gas, is the least dense and chemically the most active, displacing the other halogens from their compounds and even displacing oxygen from water. They also exhibit an almost perfect gradation of physical properties. Chemically they closely resemble one another they are nonmetallic and form monovalent negative ions.
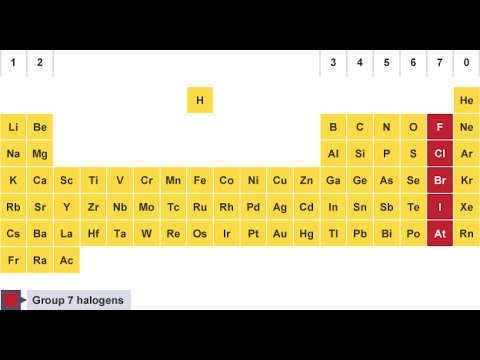
The halogens are the best-defined family of chemical elements. The chemical and physical properties of astatine are not well known it is believed to resemble iodine. Astatine (At), formerly known as alabamine, is a radioactive element also classed as a halogen its most stable isotope (which does not occur in nature) has a half-life of less than 8 1⁄2 hr. Halogen hăl´əjĕn, any of the chemically active elements found in Group 17 of the periodic table the name applies especially to fluorine (symbol F), chlorine (Cl), bromine (Br), and iodine (I).


 0 kommentar(er)
0 kommentar(er)
